Dangerously wrong oxygen readings in dark-skinned patients spur FDA scrutiny
Ars Technica » Scientific Method 2022-09-15
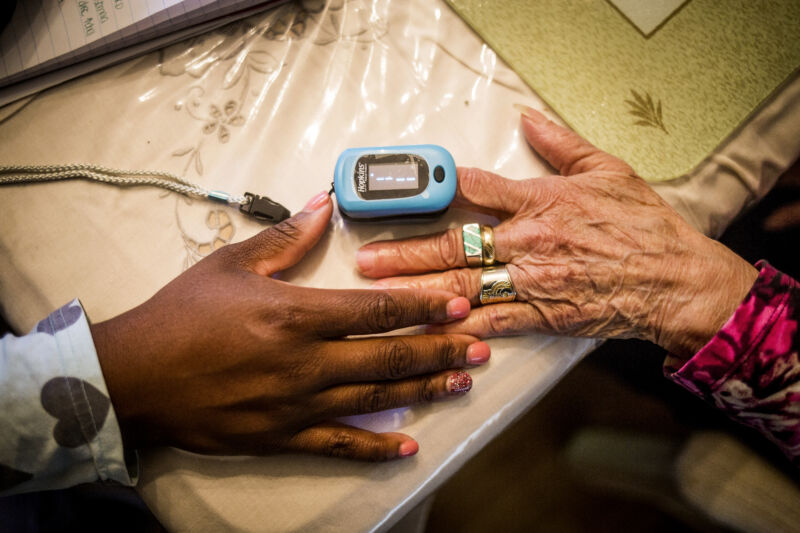
Enlarge / A nurse uses a pulse oximeter on a patient in Plainfield, New Jersey, on October 26, 2016. (credit: Getty | Bloomberg)
For years, studies have found racial bias in common oxygen measuring devices called pulse oximeters, as well as alarming dangers from inaccurate blood oxygen measurements in dark-skinned patients. Now, the US Food and Drug Administration is summoning its expert advisors to review the problematic devices and consider new recommendations and regulatory actions.
The FDA announced Thursday that its advisory committee—the Anesthesiology and Respiratory Therapy Devices Panel (ARTDP)—would convene on November 1 to discuss pulse oximeters. Until then, the agency renewed emphasis on the safety warning it issued in February 2021, which noted that the ubiquitous devices "may be less accurate in people with dark skin pigmentation."
That warning closely followed a study from December 2020 that highlighted the racial bias of pulse oximeters amid the COVID-19 pandemic. The global spread of a respiratory disease with a hallmark symptom of breathing difficulty sent pulse oximeter usage soaring—elevating the problem of racial disparities. The 2020 study—led by researchers in Michigan and published in the New England Journal of Medicine—found that pulse oximeters were nearly three times more likely to miss dangerously low blood oxygen levels (hypoxemia) in Black patients compared with white patients.