COVID-19 vaccine rollout starts in the US
Ars Technica » Scientific Method 2020-12-14
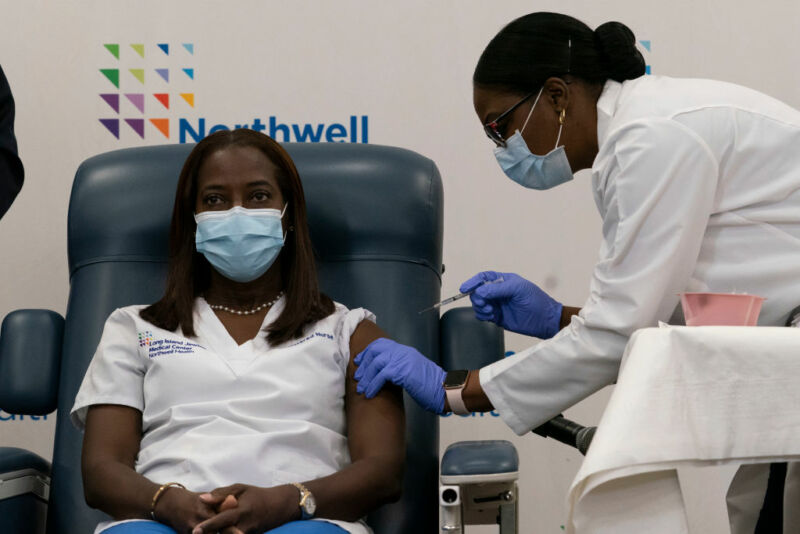
Enlarge / NEW YORK, NY - DECEMBER 14: Sandra Lindsay, left, a nurse at Long Island Jewish Medical Center, is inoculated with the COVID-19 vaccine by Dr. Michelle Chester in the Queens borough of New York City. (credit: Getty Images)
In the wake of positive results from large-scale trials, many countries are adopting the COVID-19 vaccine produced by the Pfizer/BioNTech collaboration. That led to some of the first vaccinations of the general population last week. In the US, it took until Friday for the vaccine to receive an Emergency Use Authorization from the Food and Drug Agency. After that decision, shipments of the vaccine started almost immediately, and reports are now coming in of the first vaccinations in the US.
The Pfizer/BioNTech vaccine uses a technology that, while not uncommon in biology labs, hasn't been used in vaccinations before. The vaccine consists of RNA molecules with a fatty chemical coating. The coat will fuse with the surface of human cells, dumping the RNA inside them, where it directs the production of the coronavirus's Spike protein. Once a person's cells produce Spike, the immune system reacts to it and becomes primed to protect the person from infection by the actual virus.
Unfortunately, this formulation requires that the vaccine be kept at very low temperatures during shipping. In the US, both FedEx and UPS are providing trucks equipped to handle these requirements; American and United Airlines are also collaborating to get the vaccine to distribution centers.