SCOTUS rejects challenge to abortion pill for lack of standing
Ars Technica 2024-06-13
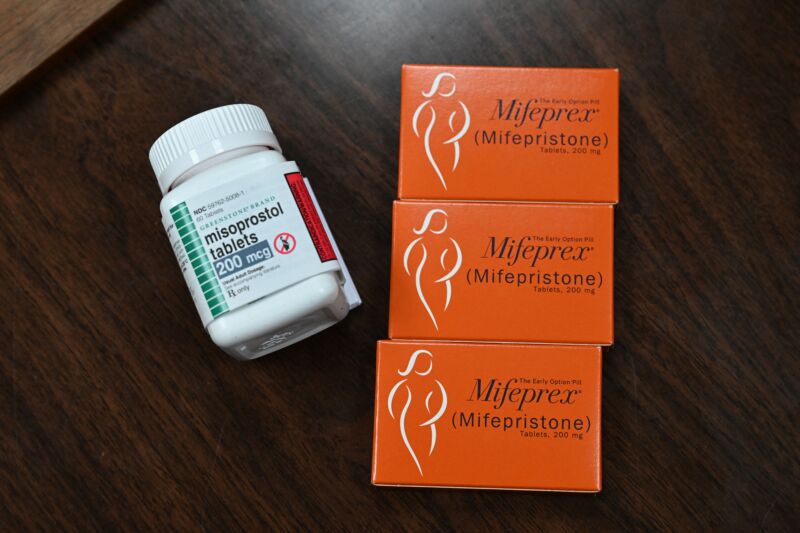
Enlarge / Mifepristone (Mifeprex) and misoprostol, the two drugs used in a medication abortion, are seen at the Women's Reproductive Clinic, which provides legal medication abortion services, in Santa Teresa, New Mexico, on June 17, 2022. (credit: Getty | Robyn Beck)
The US Supreme Court on Thursday struck down a case that threatened to remove or at least restrict access to mifepristone, a pill approved by the Food and Drug Administration for medication abortions and used in miscarriage care. The drug has been used for decades, racking up a remarkably good safety record in that time. It is currently used in the majority of abortions in the US.
The high court found that the anti-abortion medical groups that legally challenged the FDA's decision to approve the drug in 2000 and then ease usage restrictions in 2016 and 2021 simply lacked standing to challenge any of those decisions. That is, the groups failed to demonstrate that they were harmed by the FDA's decision and therefore had no grounds to legally challenge the government agency's actions. The ruling tracks closely with comments and questions the justices raised during oral arguments in March.
"Plaintiffs are pro-life, oppose elective abortion, and have sincere legal, moral, ideological, and policy objections to mifepristone being prescribed and used by others," the Supreme Court noted in its opinion, which included the emphasis on "by others." The court summarized that the groups offered "complicated causation theories to connect FDA’s actions to the plaintiffs’ alleged injuries in fact," and the court found that "none of these theories suffices" to prove harm.