Better drug made by identifying the proteins of our ancestors
Ars Technica 2016-10-08
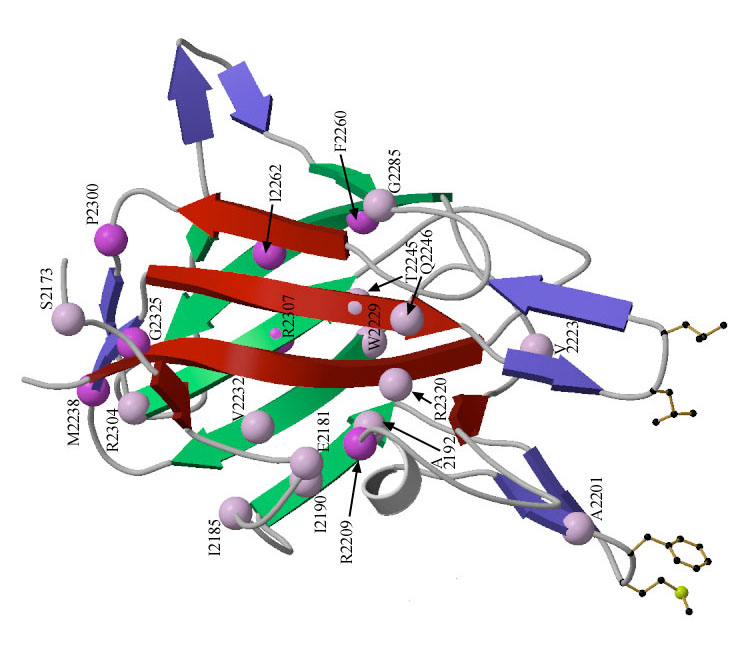
Enlarge / The structure of the human factor VIII. (credit: University of Washington)
Rational drug design seems so promising. So, you know, rational. How could it not work? Here’s the premise:
- 1. Identify a drug that has a desired effect. This is usually done either by screening a library of possible drugs or even of random chemicals, or by actively searching for a molecule hypothesized to be active against a cellular target of interest.
- 2. Figure out how the candidate drug works. What is its target is in the cell? How does it interact with said target?
- 3. Design a drug that optimizes its attraction to the target so that the final drug binds more strongly to its target, or stays bound for longer, or doesn’t accidentally also bind to something else.
Optimizing can be done by tweaking a drug’s interaction with its target: changing its shape, size, or electric charge so that it fits better. These modifications can be designed and assayed on a computer before a drug is synthesized and tested in the real world—first against a drug’s isolated target, then in cultured cells, and then animals. If all those work out, the modified drug may eventually be tested in humans.
The strategy has yielded some spectacular successes, most notably HIV protease inhibitors. But rational drug design is costly, in terms of time, money, materials, and effort. And too often it yields end products that are too toxic to be used pharmacologically, even if they bind their target well.