Bacteria under pressure run reaction in reverse to sequester carbon
Ars Technica 2017-12-28
Life performs many astonishing feats of chemistry, building complex molecules that can take us years to figure out how to synthesize. And the thermodynamics of these reactions are often fascinating—in many cases, life lives on the edge, at risk of seeing critical reactions bog down and run in reverse.
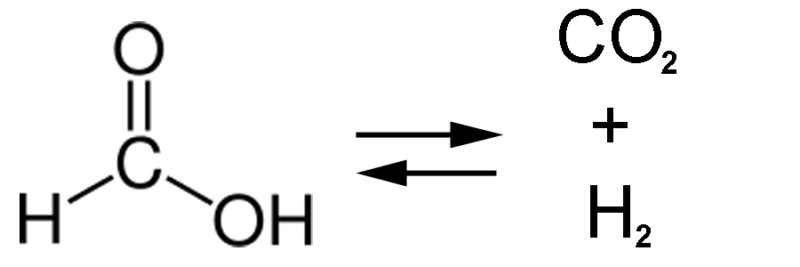
Now, some researchers have figured out a way to force bacteria to run a chemical reaction in reverse. Rather than breaking down a simple molecule into carbon dioxide, the bacteria will ingest carbon dioxide and spit out formic acid, a chemical that already has lots of uses—and could be used as fuel or to sequester carbon. The secret? Force-feed the bacteria the raw ingredients for the chemical reaction.
Enzymes and catalysis
The proteins that act as enzymes are nothing more than catalysts. The complex three-dimensional shapes of these proteins stabilize intermediate states of chemical reactions, lowering the energy required to reach them. This essentially lowers the energetic hill that has to be climbed to get between a set of reactants and a set of products. But if the overall energy of the reactants and products isn't very different, then that smaller hill will also let things run in the opposite direction: the enzyme will happily form a reaction intermediate from the product and spit out the original reactants.