The Space of Physical Frameworks (Part 1)
Azimuth 2024-09-01
Besides learning about individual physical theories, students learn different frameworks in which physical theories are formulated. I’m talking about things like this:
• classical statics • classical mechanics • quantum mechanics • thermodynamics • classical statistical mechanics • quantum statistical mechanics
A physical framework often depends on some physical constants that we can imagine varying, and in some limit one framework may reduce to another. This suggests that we should study a ‘moduli space’ or ‘moduli stack’ of physical frameworks. To do this formally, in full generality, we’d need to define what counts as a ‘framework’, and what means for two frameworks to be equivalent. I’m not ready to try that yet. So instead, I want to study an example: a 1-parameter family of physical frameworks that includes classical statistical mechanics — and, I hope, also thermodynamics!
Physicists often say things like this:
“Special relativity reduces to Newtonian mechanics as the speed of light,
approaches
”
“Quantum mechanics reduces to classical mechanics as Planck’s constant
approaches
”
“General relativity reduces to special relativity as Newton’s constant
approaches
”
Sometimes they try to elaborate this further with a picture called Bronstein’s cube or the CGh cube:
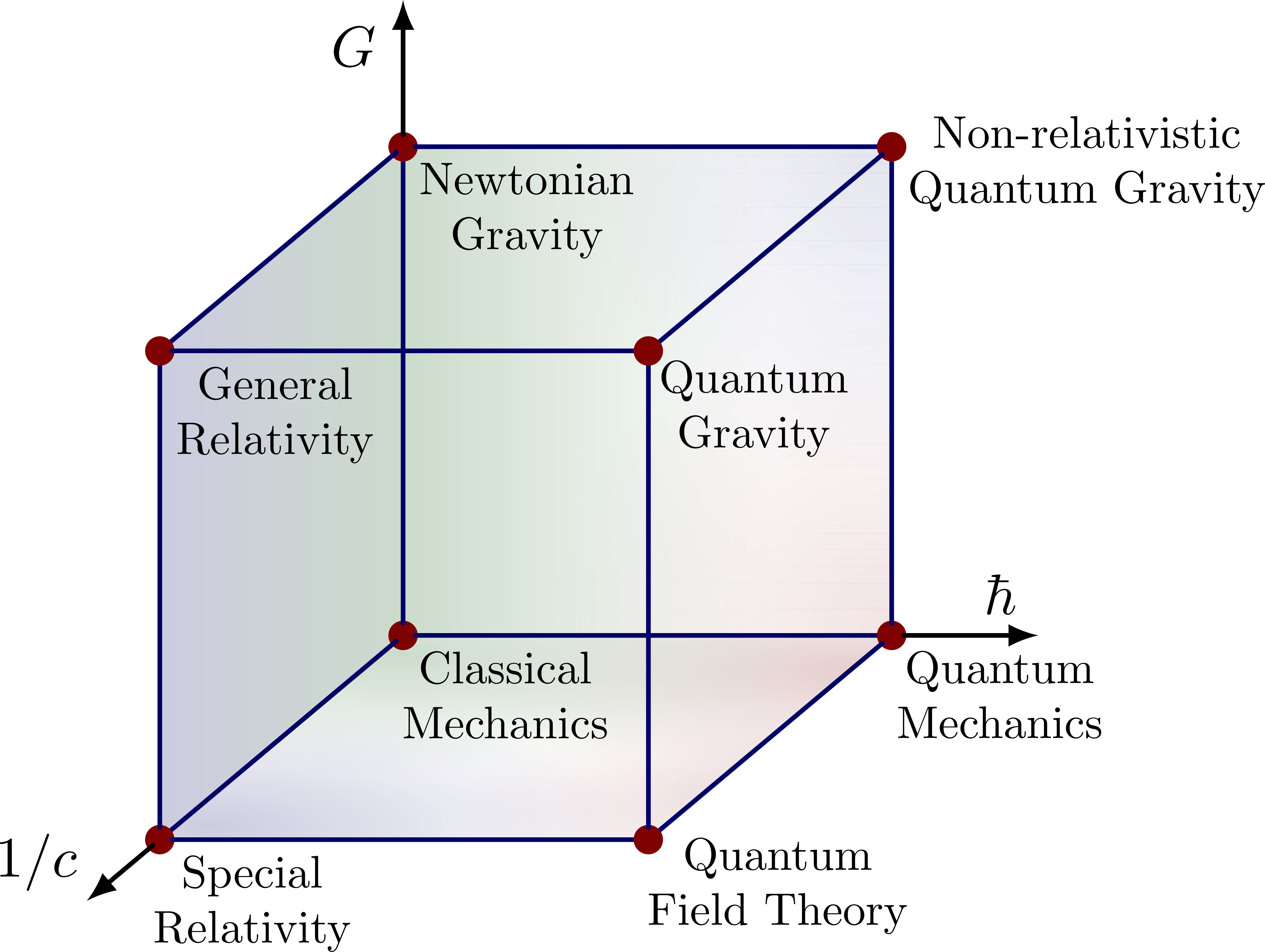
This is presumably hinting at some 3-dimensional space where and
can take arbitrary nonnegative values. This would be an example of what I mean by a ‘moduli space of physical frameworks’.
But right now I want to talk about talk about a fourth dimension that’s not in this cube. I want to talk about whether classical statistical mechanics reduces to thermodynamics as where
is Boltzmann’s constant.
Since thermodynamics and statistical mechanics are often taught in the same course, you may be wondering how I distinguish them. Here are my two key principles: anything that involves probability theory or Boltzmann’s constant I will not call thermodynamics: I will call it statistical mechanics. For example, in thermodynamics we have quantities like energy entropy
temperature
obeying rules like
But in classical statistical mechanics becomes a random variable and we instead have
In classical statistical mechanics we can also compute the variance of and this is typically proportional to Boltzmann’s constant. As
this variance goes to zero and we’re back to thermodynamics! Also, in classical statistical mechanics entropy turns out to be given by
where is some probability distribution on some measure space of states
I want to flesh out how classical statistical mechanics reduces to thermodynamics as and my hope is that this is quite analogous to how quantum mechanics reduces to classical mechanics as
:
Here’s the idea. Quantum fluctuations are a form of randomness inherent to quantum mechanics, described by complex amplitudes. Thermal fluctuations are a form of randomness inherent to classical statistical mechanics, described by real probabilities. Planck’s constant sets the scale of quantum fluctuations, and as
these go away and quantum mechanics reduces to classical mechanics. Boltzmann’s constant
sets the scale of thermal fluctuations, and as
these go away and classical statistical mechanics reduces to thermodynamics.
If this idea works, the whole story for how quantum mechanics reduces to classical mechanics as may be a Wick rotated version of how classical statistical mechanics reduces to thermodynamics as
In other words, the two stories may be formally the same if we replace
everywhere with
However, there are many obstacles to getting the idea to to work — or at least apparent obstacles, much as walls can feel like ‘obstacles’ when you’re trying to walk through a wide open door at night. Even before we meet the technical problems with Wick rotation, there’s the preliminary problem of getting thermodynamics to actually arise as the limit of classical statistical mechanics!
So despite the grand words above, it’s that preliminary problem that I’m focused on now. It’s actually really interesting.
Today I’ll just give a bit of background.
The math: deformation of rigs
Deformation quantization, is a detailed analysis of how quantum mechanics reduces to classical mechanics as and how you can try to reverse this process. If you’ve thought about this a lot, may have bumped into ‘idempotent analysis’: a version of analysis where you use minimization as a replacement for addition of real numbers, and addition as a replacement for multiplication. This works because addition distributes over minimization:
and it’s called ‘idempotent’ because minimization obeys
When we use minimization as addition we don’t get additive inverses, so numbers form a rig, meaning a ‘ring without negatives’. You also need to include to serve as an additive identity for minimization.
Idempotent analysis overlaps with ‘tropical mathematics’, where people use this number system, called the ‘tropical rig’, to simplify problems in algebraic geometry. People who do idempotent analysis are motivated more by applications to physics:
• Grigory L. Litvinov, Tropical mathematics, idempotent analysis, classical mechanics and geometry.
The basic idea is to study a 1-parameter family of rigs which for finite
are all isomorphic to
with its usual addition and multiplication, but in the limit
approach a rig isomorphic to
with ‘min’ as addition and the usual
as multiplication.
Let me describe this in more detail, so you can see exactly how it works. In classical statistical mechanics, the probability of a system being in a state of energy decreases exponentially with energy, so it’s proportional to
where is some constant we’ll discuss later. Let’s write
but let’s extend to a bijection
sending to
This says that states of infinite energy have probability zero.
Now let’s conjugate ordinary addition and multiplication, which make into a rig, by this bijection
:
These conjugated operations make into a rig. Explicitly, we have
So the multiplication is always the usual on
— yes, I know this is confusing — while the addition is some more complicated operation that depends on
We get different rig structures on
for different values of
but these rigs are all isomorphic because we got them all from the same rig structure on
However, now we can take the limit as and get operations we call
and
If we work these out we get
These give a rig structure on that’s not isomorphic to any of those for finite
This is the tropical rig.
(Other people define the tropical rig differently, using different conventions, but usually theirs are isomorphic to this one.)
The physics: classical statistical mechanics
What does all of this mean for classical statistical mechanics? The idea is that with its usual
and
is the rig of unnormalized probabilities. I’ll assume you know why we add probabilities for mutually exclusive events and multiply probabilities for independent events. But probabilities lie in
which is not closed under addition. To get a rig, we work instead with ‘unnormalized’ probabilities, which lie in
We add and multiply these just like probabilities. When we have a list of unnormalized probabilities
we can convert them to probabilities by dividing each one by their sum. We do this normalization only after all the addition and multiplication is done and we want to make predictions.
In classical statistical mechanics, a physical system has many states, each with its own energy The unnormalized probability that the system is in a state of energy
is
The case of infinite energy is not ordinarily considered, but it’s allowed by the the math here, and this gives an unnormalized probability of zero.
We can reason with these unnormalized probabilities using addition and multiplication — or, equivalently, we can work directly with the energies using the operations
and
on
In short, we’ve enhanced the usual machinery of probability theory by working with unnormalized probabilities, and then transferred it over to the world of energies.
The physical meaning of 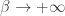
All very nice. But what’s the physical meaning of and the
limit? This is where things get tricky.
First, what’s ? In physics we usually take
where is temperature and
is Boltzmann’s constant. Boltzmann’s constant has units of energy/temperature — it’s about
joules per kelvin — so in physics we use it to convert between energy and temperature.
has units of energy, so
has units of 1/energy, and
is dimensionless. That’s important: we’re only allowed to exponentiate dimensionless quantities, and we want
to make sense.
One can imagine doing physics using some interpretation of the deformation parameter other than
But let’s take
to be
Then we can still understand the
limit in more than one way! We can
- hold
constant and let
- hold
constant and let
We could also try other things, like simply letting and
do whatever they want as long as their product approaches zero. But let’s just consider these two options.
Hold 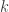 constant and let
constant and let 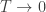
This option seems to make plenty of sense. It’s called Boltzmann’s ‘constant’, after all. So maybe we should hold it constant and let approach zero. In this case we’re taking the low temperature limit of classical statistical mechanics.
It’s sad that we get the tropical rig as a low-temperature limit: it should have been called the arctic rig! But the physics works out well. At temperature systems in classical statistical mechanics minimize their free energy
where
is energy and
is entropy. As
free energy reduces to simply the energy,
Thus, in the low temperature limit, such systems always try to minimize their energy! In this limit we’re doing classical statics: the classical mechanics of systems at rest.
These ideas let us develop this analogy:
Blake Pollard and I explored this analogy extensively, and discovered some exciting things:
• Quantropy:
o Part 1: the analogy between quantum mechanics and statistical mechanics, and the quantum analogue of entropy: quantropy.
o Part 2: computing the quantropy of a quantum system starting from its partition function.
o Part 3: the quantropy of a free particle.
o Part 4: a paper on quantropy, written with Blake Pollard.
But while this analogy is mathematically rigorous and leads to new insights, it’s not completely satisfying. First, and
just feel different. Planck’s constant takes the same value everywhere while temperature is something we can control. It would be great if next to the thermostat on your wall there was a little box where you could adjust Planck’s constant, but it just doesn’t work that way!
Second, there’s a detailed analogy between classical mechanics and thermodynamics, which I explored here:
• Classical mechanics versus thermodynamics:
o Part 1: Hamilton’s equations versus the Maxwell relations.
o Part 2: the role of symplectic geometry.
o Part 3: a detailed analogy between classical mechanics and thermodynamics.
o Part 4: what is the analogue of quantization for thermodynamics?
Most of this was about how classical mechanics and thermodynamics share common mathematical structures, like symplectic and contact geometry. These structures arise naturally from variational principles: principle of least action in classical mechanics, and the principle of maximum entropy in thermodynamics. By the end of this series I had convinced myself that thermodynamics should appear as the limit of some physical framework, just as classical mechanics appears as the
limit of quantum mechanics. So, let’s look at option 2.
Hold 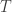 constant and let
constant and let 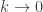
Of course, my remark about holding constant because it’s called Boltzmann’s constant was just a joke. Planck’s constant is a constant too, yet it’s very fruitful to imagine treating it as a variable and letting it approach zero.
But what would it really mean to let ? Well, first let’s remember what it would mean to let
I’ll pick a specific real-world example: a laser beam. Suppose you’re a physicist from 1900 who doesn’t know quantum mechanics, who is allowed to do experiments on a beam of laser light. If you do crude measurements, this beam will look like a simple solution to the classical Maxwell equations: an electromagnetic field oscillating sinusoidally. So you will think that classical physics is correct. Only when you do more careful measurements will you notice that this wave’s amplitude and phase are a bit ‘fuzzy’: you get different answers when you repeatedly measure these, no matter how good the beam is and how good your experimental apparatus is. These are ‘quantum fluctuations’, related to the fact that light is made of photons.
The product of the standard deviations of the amplitude and phase is bounded below by something proportional to So, we say that
sets the scale of the quantum fluctuations. If we could let
these fluctuations would become ever smaller, and in the limit the purely classical description of the situation would be exact.
Something similar holds with Boltzmann’s constant. Suppose you’re a physicist from 1900 who doesn’t know about atoms, who has somehow been given the ability to measure the pressure of a gas in a sealed container with arbitrary accuracy. If you do crude measurements, the pressure will seem to be a function of the temperature, the volume of the container, and the amount of gas in the container. This will obey the laws of thermodynamics. Only when you do extremely precise experiments will you notice that the pressure is fluctuating. These are ‘thermal fluctuations’, caused by the gas being made of molecules.
The variance of the pressure is proportional to So, we say that
sets the scale of the thermal fluctuations. If we could let
these fluctuations would become ever smaller, and in the limit the thermodynamics description of the situation would be exact.
The analogy here is a bit rough at points, but I think there’s something to it. And if you examine the history, you’ll see some striking parallels. Einstein discovered that light is made of photons: he won the Nobel prize for his 1905 paper on this, and it led to a lot of work on quantum mechanics. But Einstein also wrote a paper in 1905 showing how to prove that liquid water is made of atoms! The idea was to measure the random Brownian motion of a grain of pollen in water — that is, thermal fluctuations. In 1908, Jean Perrin carried out this experiment, and he later won the Nobel “for his work on the discontinuous structure of matter”. So the photon theory of light and the atomic theory of matter both owe a lot to Einstein’s work.
Planck had earlier introduced what we now call Planck’s constant in his famous 1900 paper on the quantum statistical mechanics of light, without really grasping the idea of photons. Remarkably, this is also the paper that first introduced Boltzmann’s constant Boltzmann had the idea that entropy was proportional to the logarithm of the number of occupied states, but he never estimated the constant of proportionality or gave it a name: Planck did both! So Boltzmann’s constant and Planck’s constant were born hand in hand.
There’s more to say about how to correctly take the limit of a laser beam: we have to simultaneously increase the expected number of photons, so that the rough macroscopic appearance of the laser beam remains the same, rather than becoming dimmer. Similarly, we have to be careful when taking the
limit of a container of gas: we need to also increase the number of molecules, so that the average pressure remains the same instead of decreasing.
Getting these details right has exercised me quite a bit lately: I’m trying to develop a general theory of the limit, but it’s been essential to work through some examples. I’ll talk about one next time.