Efficient OLED gets rid of heavy metals
Ars Technica » Scientific Method 2012-12-14
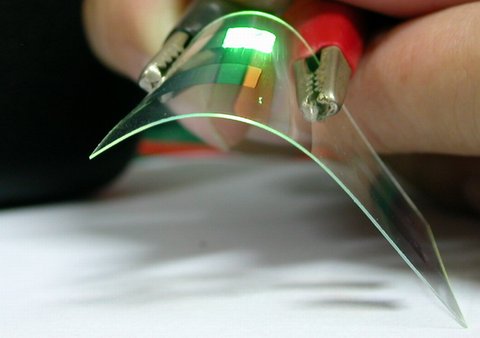
A new molecule increases the efficiency of fluorescent organic light emitting diodes without using heavy metals. Early OLEDs were made from fluorescent molecules that transform added electrical energy into light. However, rules of quantum mechanics make this transformation rather inefficient.
Here’s why. Pumping electricity through a fluorescent OLED excites charge carriers—electrons and positively charged “holes”—in the molecules. These charge carriers meet to form a bound state called an exciton. This exciton can be one of two types: a singlet exciton or a triplet exciton. That energy has to be released, but only singlet excitons can release it as light, and singlets only occur 25 percent of the time. The other 75 percent of excitons are triplets that relax by releasing heat, not light.
To increase the efficiency of these OLEDs, scientists add heavy metal atoms to help the previously heat-producing states emit light—with nearly 100 percent efficiency—through phosphorescence.
Read 6 remaining paragraphs | Comments